Light is composed of electromagnetic waves come in a spectrum of different wavelengths, from radio waves to visible light to X-rays. Our eyes can only see some of these wavelengths: we call the electromagnetic waves we see visible light, and the different wavelengths we see different colours. Light comes in packets called photons: the wavelength of the light determines how much energy a single packet of light has. In visible light, blue has the shortest wavelength and highest energy, while red has the longest wavelength and lowest energy.

Electromagnetic Spectrum
(hosted by Wikimedia Commons)
Often, when a telescope takes a picture of an astronomical object, a filter is used to only allow certain wavelengths of light through. If you imagine a red stained glass window, it acts as a filter only letting red light through: sunlight composed of many different wavelengths hits the window from outside, but only the red wavelengths of light make it through the window to be seen inside. But why would astronomers only want to look at specific wavelengths of light?
The answer has to do with the structure of atoms. Atoms are composed of a nucleus surrounded by electrons, and the electrons are only allowed in certain energy levels. To move to a higher energy level, the atom must absorb a photon; similarly, to move to a lower energy level, the atom must release a photon. Moving between any specific two energy levels requires absorbing or releasing a photon of a specific energy, and thus involves light of a specific wavelength (or equivalently, specific colour). Atoms can also have one of their electrons removed by a photon in a process called photoionization. An atom can only be photoionized by a photon with an energy higher than some threshold, and thus light that has a shorter wavelength than some threshold (or equivalently, light bluer than some threshold). Thus, we can learn what the atoms in the Eagle Nebula are doing by looking at the specific wavelengths of light that they emit.
Bohr Model of the Atom
(hosted by Wikimedia Commons)
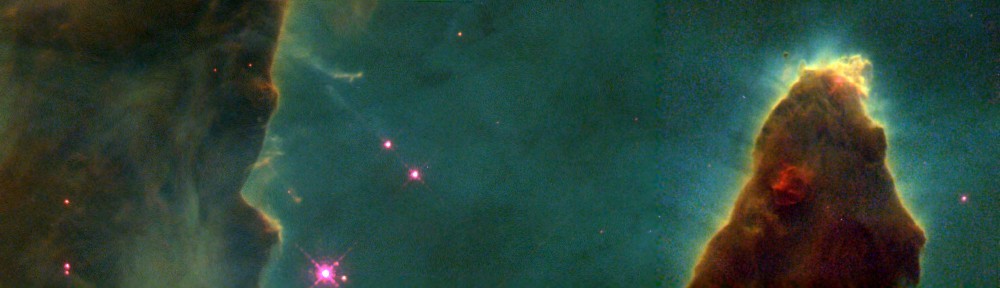
Take a first look at the “Pillars of Creation” to learn about the specific wavelengths of light that were observed to make “The Pillars of Creation” and what they tell us about the nebula.